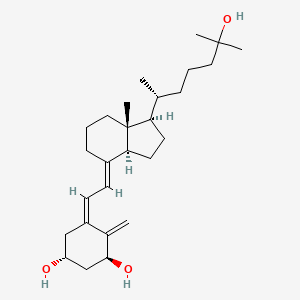
calcitriol, Rocaltrol, Calcijex, 32222-06-3, 1alpha,25-Dihydroxyvitamin D3, Topitriol, Silkis, 1alpha,25-Dihydroxycholecalciferol, Soltriol, Vectical, 1,25-DHCC, Calcitriolum, 1,25-DIHYDROXYCHOLECALCIFEROL, 1,25-Dihydroxyvitamin D, 1,25-Dihydroxyvitamin D3, 1alpha,25(OH)2D3, 1alpha,25-Dihydroxyvitamin D, 1-alpha,25-Dihydroxyvitamin D3, Ro 21-5535, Dihydroxyvitamin D3, 1,25-Dihydroxycholecaliferol, CCRIS 5522, Panbonis, HSDB 3482, Calcitriolum [INN-Latin], UNII-FXC9231JVH, EINECS 250-963-8, FXC9231JVH, DN-101, 1,25-dihydroxy vitamin D3, 1,25 Dihydroxycholecalciferol, Ro 215535, CHEBI:17823, (1S,3R,5Z,7E)-9,10-secocholesta-5,7,10-triene-1,3,25-triol, 1-alpha-25-dihydroxyvitamin D3, CHEMBL846, U 49562, Ro-215535, 1a,25-Dihydroxyvitamin D3, Cholecalciferol, 1-alpha,25-dihydroxy-, Ro-21-5535, 1a,25-Dihydroxycholecalciferol, DTXSID5022722, vit D, (5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene-1alpha,3beta,25-triol, 9,10-Seco(5Z,7E)-5,7,10(19)-cholestatriene-1alpha,3beta,25-triol, (1alpha,3beta,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol, (5Z,7E)-(1S,3R)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol, 9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1alpha,3beta,5Z,7E)-, MFCD00867079, (1R,3S,Z)-5-((E)-2-((1R,3aS,7aR)-1-((R)-6-hydroxy-6-methylheptan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol, Calcitriol [USAN:USP:INN:BAN:JAN], DN 101, (1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol, Calcitriolum (INN-Latin), CALCITRIOL (MART.), CALCITRIOL [MART.], CALCITRIOL (USP-RS), CALCITRIOL [USP-RS], Toptriol, (1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(2R)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol, 1,25-DIHYDROXYCHOLECALCIFEROL, (1ALPHA)-, 1000873-74-4, CALCITRIOL (EP MONOGRAPH), CALCITRIOL [EP MONOGRAPH], Decostriol, CALCITRIOL (USP MONOGRAPH), CALCITRIOL [USP MONOGRAPH], (1S,3R,5Z,7E)-9,10-seco-5,7,10(19)-cholestatriene-1,3,25-triol, (5Z,7E)-(1S,3R)-9,10-seco-5,7,10(19)-cholestatriene-1,3,25-triol, Calcitriol (USAN:USP:INN:BAN:JAN), 1alpha,25-dihydroxyvitamin D3 / 1alpha,25-dihydroxycholecalciferol / calcitriol, SMR000466393, 1,25 (OH)2 D3, dihydroxy-vitamin D3, 1 alpha,25-Dihydroxyvitamin D3, DTXCID002722, 25-dihydroxycholecalciferol, 1,25 Dihydroxyvitamin D3, calcitriolo, 1,25-(OH)2D3, Asentar, Calcitrol, D3, 1,25-Dihydroxyvitamin, 1,25-(OH)2-D3, 1.alpha.,25-dihydroxycholecalciferol, 9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1a,3b,5Z,7E)-, 1 alpha,25 Dihydroxyvitamin D3, Calcitriol; (5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene-1alpha,3beta,25-triol, 9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1.alpha.,3.beta.,5Z,7E)-, 1alpha 25-dihydroxycholecalciferol, D3, 1 alpha,25-Dihydroxyvitamin, Rocaltrol (TN), NCGC00161327-04, (1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(1R)-5-hydroxy-1,5-dimethyl-hexyl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylene-cyclohexane-1,3-diol, 5-{2-[1-(5-HYDROXY-1,5-DIMETHYL-HEXYL)-7A-METHYL-OCTAHYDRO-INDEN-4-YLIDENE]-ETHYLIDENE}-4-METHYLENE-CYCLOHEXANE-1,3-DIOL, 1 alpha,25-Dihydroxycholecalciferol, Calcitriol 0.25 mcg, 1db1, starbld0021993, CALCITRIOL [MI], 1, 25-(OH)2D3, CALCITRIOL [INN], CALCITRIOL [JAN], Spectrum5_002061, CALCITRIOL [HSDB], CALCITRIOL [USAN], CALCITRIOL [VANDF], SCHEMBL3245, CALCITRIOL [WHO-DD], 1alpha,25(OH)2-D3, BSPBio_001287, Calcitriol (JAN/USP/INN), Calcitriol Capsules 0.5 mcg, MLS000759536, MLS001424122, Calcitriol Capsules 0.25 mcg, BML2-E03, GTPL2779, CALCITRIOL [ORANGE BOOK], BCBcMAP01_000160, CHEBI:93988, A11CC04, Cas Number 32222-06-3, D05AX03, BCPP000304, HMS1361A09, HMS1791A09, HMS1989A09, HMS2051F06, HMS2089N03, HMS2232D18, HMS3402A09, 1 alpha ,25-Dihydroxyvitamin D3, 5-(2-(1-(5-HYDROXY-1,5-DIMETHYL-HEXYL)-7A-METHYL-OCTAHYDRO-INDEN-4-YLIDENE)-ETHYLIDENE)-4-METHYLENE-CYCLOHEXANE-1,3-DIOL, EX-A4435, 1,25 DIHYDROXY VITAMIN D3, 1a,25-(OH)2D3, BDBM50200182, LMST03020258, NSC749776, s1466, 1-alpha,-1,25-Dihydroxyvitamin D3, AKOS015961898, AC-1859, BCP9000474, CCG-101001, CD-2027, CS-0388, DB00136, NC00251, NSC-749776, 1,25(OH2)D3, IDI1_033757, 1,25(OH)2D3 & CD4, Calcitriol (1,25-Dihydroxyvitamin D3), Calcitrol 100 microg/mL in Acetonitrile, NCGC00161327-01, (1R,3S,Z)-5-(2-((1R,3aS,7aR,E)-1-((R)-6-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol, 1,25(OH)2-D3, 1.alpha.,25-Dihydroxyvitamin D(sub 3), 66772-14-3, CPD000466393, HY-10002, C3078, FT-0607271, NS00004734, Vitamin D3-1alpha,25-dihydroxy (Calcitriol), 1,25D3, C01673, D00129, AB00639957-06, AB00639957_07, 1alpha,25-Dihydroxyvitamin D3, >=99% (HPLC), EN300-22411582, Q139195, SR-01000759361, SR-01000946978, 1alpha,25-Dihydroxyvitamin D3, >=97.0% (HPLC), SR-01000759361-4, SR-01000946978-1, 1.Alpha.,25-Dihydroxy-26,27-hexadeuterovitamin D3, BRD-K27316855-001-06-7, BRD-K27316855-001-19-0, (3b,5Z,7E)-9,10-Secocholesta-5,7,10(19)-trienetriol, Calcitriol, European Pharmacopoeia (EP) Reference Standard, (5Z,10-secocholesta-5,7,10(19)-triene-1.alpha.,3.beta.,25-triol, (1S,3R,5Z,7E,14beta,17alpha)-9,10-secocholesta-5,7,10-triene-1,3,25-triol, 9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1.alpha.,3.beta,.5Z,7E)-, 9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1.alpha.,3.beta,.5Z,7E)- & CD4, (1R,3S)-5-{2-[(1R,3aS,7aR)-1-((R)-5-Hydroxy-1,5-dimethyl-hexyl)-7a-methyl-octahydro-inden-4-ylidene]-ethylidene}-4-methylene-cyclohexane-1,3-diol, (1R,3S,5Z)-5-{2-[(1R,3aS,4E,7aR)-1-[(2R)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-octahydro-1H-inden-4-ylidene]ethylidene}-4-methylidenecyclohexane-1,3-diol, 1,3-Cyclohexanediol, 4-methylene-5-[2-[(1R,3aS,7aR)-octahydro-1-[(1R)-5-hydroxy-1,5-dimethylhexyl]-7a-methyl-4H-inden-4-ylidene]ethylidene]-, (1R,3S)-